Dinuclear nickel(ii) complexes with 2,5-diamino-1,4-benzoquinonediimine ligands as precatalysts for the polymerization of styrene: electronic and steric substituent effects†
Abstract
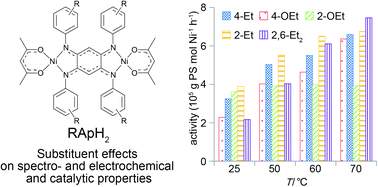
Catalytic polymerization of styrene with a series of dinuclear nickel(II) complexes [{Ni(acac)}2{μ-C6H2-(N-Ph-R)4}] (R = 4-Et (1a), 4-OEt (1b), 2-OEt (1c), 2-Et (1d), and 2,6-Et2 (1e)) in the presence of methylaluminoxane was studied under various conditions to evaluate the substituent effect. The activity of 1a–1e, except 1c, increased with an increase in the reaction temperature, and the highest activity (7.46 × 105 g PS mol−1 Ni h−1) was obtained using 1e at 70 °C. The electronic and steric properties of the ligand influenced the activity at room temperature, 50 °C, and 60 °C, steric factors barely, whereas electronic factors slightly dominated the activity at 70 °C. The activity with 1c remained constant at all temperatures, probably due to the masking of the active center by the formation of inactive N,N′,O-chelate species. The obtained polymers were atactic polystyrenes with molecular weights and molecular weight distributions in the range of 15 000–43 400 and 1.71–2.14, respectively. While a clear dependence of the molecular weight and molecular weight distribution on the temperature was observed, no significant dependence on the substituent was noted.

 Please wait while we load your content...
Please wait while we load your content...