Application of three-coordinate copper(i) complexes with halide ligands in organic light-emitting diodes that exhibit delayed fluorescence†
Abstract
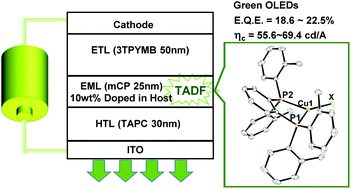
A series of three-coordinate copper(I) complexes (LMe)CuX [X = Cl (1), Br (2), I (3)], (LEt)CuBr (4), and (LiPr)CuBr (5) [LMe = 1,2-bis[bis(2-methylphenyl)phosphino]benzene, LEt = 1,2-bis[bis(2-ethylphenyl)phosphino]benzene, and LiPr = 1,2-bis[bis(2-isopropylphenyl)phosphino]benzene] exhibit efficient blue-green emission in the solid state at ambient temperature with peak wavelengths between 473 and 517 nm. The emission quantum yields were 0.38–0.95. The emission lifetimes were measured in the temperature range of 77–295 K using a nanosecond laser technique. The temperature dependence of the emission lifetimes was explained using a model with two excited states: a singlet and a triplet state. The small energy gaps (<830 cm−1) between the two states suggest that efficient emission from 1–5 was thermally activated delayed fluorescence (TADF). Alkyl substituents at ortho positions of peripheral phenyl groups were found to have little effect on the electronic excited states. Because the origin of the emission of complexes 2, 4, and 5 was thought to be a (σ + Br)→π* transition, photoluminescence characteristics of these complexes were dominated by the diphosphine ligands. Complexes 2, 4, and 5 had similar emission properties. Complexes 1–5 had efficient green TADF in amorphous films at 293 K with maximum emission wavelengths of 508–520 nm and quantum yields of 0.61–0.71. Organic light-emitting devices that contained complexes 1–5 and exhibited TADF exhibit bright green luminescence with current efficiencies of 55.6–69.4 cd A−1 and maximum external quantum efficiencies of 18.6–22.5%.
- This article is part of the themed collection: Luminescent Complexes and Materials for Light-Emitting Devices

 Please wait while we load your content...
Please wait while we load your content...