Chemical reduction of the elastic properties of zeolites: a comparison of the formation of carbonate species versus dealumination†
Abstract
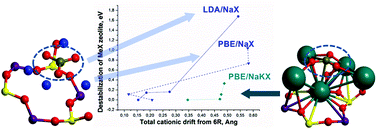
The decrease in elastic moduli (Young's, bulk, and shear modulus), the variations in their asymmetries, the Poisson's ratio and the linear compressibility due to carbonate formation in NaX, have been compared to those produced by dealumination of the zeolite HY framework, from the Al–Si–Al fragment positioned in joined 4R rings. All these systems have been considered at the density functional theory (DFT) level using periodic boundary conditions. The representativeness of the models has been checked by comparison of the calculated IR spectra of carbonate and hydrocarbonate species in NaX and of hydroxyl groups in HY with the experimental equivalents. The correlation between the destabilization energy of the systems and the displacement of Na or K cations coordinated to the carbonate or hydrocarbonate species, expressed in terms of Me–O bond elongation, has been confirmed for either one or two carbonate and hydrocarbonate species per unit cell (UC). Finally, a similar reduction in elasticity in FAU zeolites has been observed, due either to carbonate/bicarbonate formation in NaX or as a step in HY dealumination.

 Please wait while we load your content...
Please wait while we load your content...