Enhancing σ/π-type copper(i)⋯thiophene interactions by metal doping (metal = Li, Na, K, Ca, Sc)†
Abstract
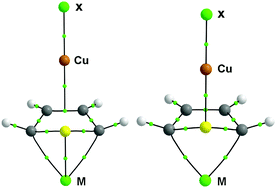
The influence of metal doping on σ/π-type copper(I)⋯thiophene interactions and the nature of Cu⋯π/S bonding have been investigated. Our calculated results show that Li, Na, K, Ca and Sc atom doping on thiophene enhances the copper(I)⋯thiophene interactions. Enhancement factors are determined by the electrostatic potential of the thiophene molecular surface and the electronic configuration of the doping metal. The more negative the electrostatic potential, the stronger is the interaction. The influence of the d-block transition metal element (Sc) is larger than that of s-block main group metal elements. Both the σ and π type Cu⋯thiophene interactions are of moderate strengths and display partial covalent characters. Linear relationships exist between the topological properties (ρ(rc), ∇2ρ(rc), δ(A, B) and Hc) at the BCP and the bond lengths d(Cu⋯π/S). When the Cu⋯π/S bond length became shorter, larger ∇2ρ(rc), δ(A, B) and smaller Hc values can be predicted, resulting in greater covalent character of Cu⋯π/S bonding.

 Please wait while we load your content...
Please wait while we load your content...