Alkali-metal ion coordination in uranyl(vi) poly-peroxide complexes in solution. Part 1: the Li+, Na+ and K+ – peroxide–hydroxide systems†
Abstract
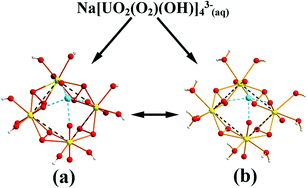
The alkali metal ions Li+, Na+ and K+ have a profound influence on the stoichiometry of the complexes formed in uranyl(VI)–peroxide–hydroxide systems, presumably as a result of a templating effect, resulting in the formation of two complexes, M[(UO2)(O2)(OH)]2− where the uranyl units are linked by one peroxide bridge, μ–η2–η2, with the second peroxide coordinated “end-on”, η2, to one of the uranyl groups, and M[(UO2)(O2)(OH)]43−, with a four-membered ring of uranyl ions linked by μ–η2–η2 peroxide bridges. The stoichiometry and equilibrium constants for the reactions: M+ + 2UO22+ + 2HO2− + 2H2O → M[(UO2)(O2)(OH)]2− + 4H+ (1) and M+ + 4UO22+ + 4HO2− + 4H2O → M[(UO2)(O2)(OH)]43− + 8H+ (2) have been measured at 25 °C in 0.10 M (tetramethyl ammonium/M+)NO3 ionic media using reaction calorimetry. Both reactions are strongly enthalpy driven with large negative entropies of reaction; the observation that ΔH(2) ≈ 2ΔH(1) suggests that the enthalpy of reaction is approximately the same when peroxide is added in bridging and “end-on” positions. The thermodynamic driving force in the reactions is the formation of strong peroxide bridges and the role of M+ cations is to provide a pathway with a low activation barrier between the reactants and in this way “guide” them to form peroxide bridged complexes; they play a similar role as in the synthesis of crown-ethers. Quantum chemical (QC) methods were used to determine the structure of the complexes, and to demonstrate how the size of the M+-ions affects their coordination geometry. There are several isomers of Na[(UO2)(O2)(OH)]2− and QC energy calculations show that the ones with a peroxide bridge are substantially more stable than the ones with hydroxide bridges. There are isomers with different coordination sites for Na+ and the one with coordination to the peroxide bridge and two uranyl oxygen atoms is the most stable one.

 Please wait while we load your content...
Please wait while we load your content...