Abstract
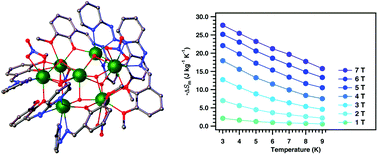
The reaction of the hydrazone, 2-methoxy-6-(pyridin-2-yl-hydrazonomethyl) phenol (LH) with lanthanide(III) nitrate salts in the presence of excess triethylamine afforded the heptanuclear Ln(III) complexes: [Gd7(L)6(μ3-OH)8(NO3)4(H2O)]·(NO3)3·8CH3CN·H2O (1), [Tb7(L)6(μ3-OH)8(NO3)4]·(NO3)3·9CH3CN·2CH3OH·3H2O (2), [Dy7(L)6(μ3-OH)8(NO3)4(H2O)]·(NO3)3·7CH3CN·3H2O (3), [Ho7(L)6(μ3-OH)8(NO3)4]·(NO3)3·11CH3CN·2CH3OH·2H2O (4) and [Er7(L)6(μ3-OH)8(NO3)4]·(NO3)3·8CH3CN·2CH3OH (5). Single crystal X-ray diffraction studies reveal that these complexes are tri-cationic, possessing three nitrate counter anions. The heptanuclear ensemble is non-planar and consists of six [L]− and eight μ3-OH ligands. These compounds show an interesting structural motif with two incomplete cubes fused to each other through a common Ln(III) ion. Compound 1 exhibits a magnetocaloric effect, with (−ΔSm(T) = 27.7 J kg−1 K−1 at 3 K and under a field change of 0–7 T), while compound 3 shows slow magnetic relaxation at very low temperatures.


 Please wait while we load your content...
Please wait while we load your content...