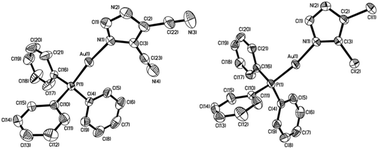
A study on the inhibition of dihydrofolate reductase (DHFR) from Escherichia coli by gold(i) phosphane compounds. X-ray crystal structures of (4,5-dichloro-1H-imidazolate-1-yl)-triphenylphosphane-gold(i) and (4,5-dicyano-1H-imidazolate-1-yl)-triphenylphosphane-gold(i)†
Abstract
An unprecedented study on the inhibitory activities of a class of phosphane gold(I) complexes on E. coli dihydrofolate reductase (DHFR) is reported. The gold(I) complexes considered in this work consist of azolate or chloride ligands and phosphane as co-ligands. The ligands have been functionalized with polar groups (–COOH, –COO−, NO2, Cl, CN) to obtain better solubility in polar media. Neutral, anionic and cationic gold(I) complexes have been tested as DHFR inhibitors by means of a continuous direct spectrophotometric method. X-ray structural characterizations were performed on ((triphenylphosphine)-gold(I)-(4,5-dicyanoimidazolyl-1H-1yl) and on the analog (triphenylphosphine)-gold(I)-(4,5-dichloroimidazolyl-1H-1yl). The inhibition constants obtained from the enzyme tests range from 20 μM to 63 nM (auranofin) and are conducive to promoting these compounds as potential DHFR inhibitors.

 Please wait while we load your content...
Please wait while we load your content...