Catalytic upgrading of α-angelica lactone to levulinic acid esters under mild conditions over heterogeneous catalysts†
Abstract
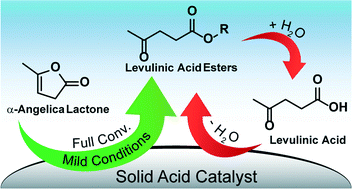
Butyl levulinate was prepared starting from α-angelica lactone and butanol over Amberlyst® 36. Different reaction conditions were optimized, which resulted in full conversion and 94% selectivity toward the ester at 75 °C. A reaction network analysis reveals pseudo-butyl levulinate and levulinic acid as intermediates in the preparation of butyl levulinate. The mild protocol was successfully applied for different alcohols and compared with the esterification of levulinic acid. Overall, this study identifies α-angelica lactone as a better candidate than levulinic acid for the heterogeneously catalysed preparation of levulinic acid esters. A catalyst screening shows that also zeolites and zirconia-based catalysts are able to catalyse the reaction. However, the transformation of the intermediate pseudo-butyl levulinate into butyl levulinate requires acid sites of sufficient strength to proceed.

 Please wait while we load your content...
Please wait while we load your content...