Nickel and cobalt phosphides as effective catalysts for oxygen removal of dibenzofuran: role of contact time, hydrogen pressure and hydrogen/feed molar ratio†
Abstract
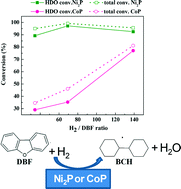
The catalytic activity of nickel and cobalt phosphides, with a metal loading of 5 wt.%, supported on silica was investigated in the hydrodeoxygenation reaction (HDO) of dibenzofuran (DBF) as a model oxygenated compound at different contact times, H2 pressures and H2/DBF molar ratios. The aim of the study was to understand the mechanism of the reaction and to study the impact of H2 pressure and H2/DBF molar ratio on the reaction. The catalysts were characterized by N2 adsorption–desorption isotherm measurement at −196 °C, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), CO chemisorption, NH3 Temperature-Programmed Desorption (NH3-TPD), IR spectroscopy and H2 Temperature-Programmed Desorption (H2-TPD). The prepared catalysts were tested in the HDO reaction of DBF in a continuous-flow fixed-bed stainless steel catalytic reactor at pressures ranging from 1–30 bar at 275 °C. The results obtained indicate that the Ni2P catalyst is more active than the CoP catalyst, converting more than 90% of DBF at the highest contact time into oxygen-free products. The activity of both catalysts increases with increased contact time. At low contact times, the intermediates tetrahydrodibenzofuran (THDBF) and hexahydrodibenzofuran (HHDBF) are observed as products, while an increment in the contact time led to the transformation of THDBF and HHDBF into O-free compounds, mainly bicyclohexane (BCH), indicating that the HDO of DBF follows the path: DBF → HHDBF → THDBF → 2-CHP → BCH. Further, both Ni2P and CoP catalysts are active at medium pressures with HDO degrees similar to those obtained at 30 bar. Ni2P is less affected by the changes in H2/DBF ratio than CoP and the catalysts are more active at high H2/DBF molar ratios.

 Please wait while we load your content...
Please wait while we load your content...