n-Bu4NI-catalyzed direct amination of ethers with aryl tetrazoles and triazoles via cross-dehydrogenative coupling reaction†
Abstract
An efficient, metal-free protocol for direct amination of ethers with aryl tetrazoles and triazoles has been developed using tetrabutylammonium iodide (TBAI) as a catalyst and tert-butyl hydroperoxide (t-BuOOH) as an oxidant. A series of ethers, aryl tetrazoles as well as triazoles are tolerated in this system, giving the corresponding products in moderate to good yields.
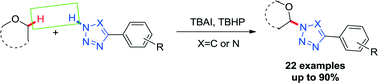

 Please wait while we load your content...
Please wait while we load your content...