Significant effect of base on the improvement of selectivity in the hydrogenation of benzoic acid over NiZrB amorphous alloy supported on γ-Al2O3†
Abstract
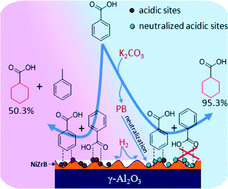
This study presents a facile way to improve the selectivity for cyclohexanecarboxylic acid by adding a base in the hydrogenation of benzoic acid over a non-noble metal NiZrB amorphous alloy supported on γ-Al2O3. It is found that alkali metal carbonates exhibit an excellent selectivity improvement from 50.3% to a range of 93.5–95.7%, with the conversion of benzoic acid higher than 92.3%. Even a very small amount of K2CO3 (1 mol% benzoic acid) was efficient for improving the selectivity for cyclohexanecarboxylic acid. In addition, a lower reaction temperature was beneficial to the improvement of selectivity. Based on the results of temperature programmed desorption of NH3 and inductively coupled plasma analysis, the improvement of selectivity in the presence of a base was attributed to the neutralization of the acidic sites on the surface of the catalyst by the in situ generated potassium benzoate, inhibiting the hydrodeoxygenation of carbonyl and resulting in a high selectivity for cyclohexanecarboxylic acid.

 Please wait while we load your content...
Please wait while we load your content...