Study on the one-pot oxidative esterification of glycerol with MOF supported polyoxometalates as catalyst†
Abstract
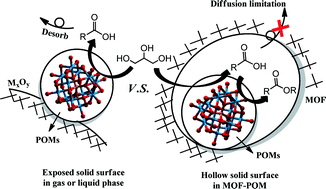
In this work, glycerol was treated under green and mild conditions (water solvent, H2O2 oxidant, 40 °C) in an attempt to utilise its additional value. With a metal organic framework (MOF) supported polyoxometallate (POM) as a catalyst, esters were generated as one of the major products which could be useful for various industrial applications. The selectivity of esters formation reached 34.5% in this one-pot oxidative esterification process. Benefiting from the pore limitation effect of the MOF, diffusion was restricted and the original products could be further transformed into esters with the existence of the POM. No other reagents were needed during this process, and all of the intermediates were produced from glycerol itself. The oxidative esterification reaction was studied in detail including the role of the MOF, the influence of pH and the POM type, the mechanism and so on. It was concluded that the POM served as the active site for this oxidative esterification process and H2O2 provided weak acidity in addition to the source of oxygen. Too stronger acidity and oxidizability were unfavourable to the generation of esters. Also, the catalysts could be recovered after reaction, exhibiting good stability and reusability.

 Please wait while we load your content...
Please wait while we load your content...