Desulfitative Pd-catalysed coupling reaction using benzenesulfonyl chlorides and enones as the coupling partners
Abstract
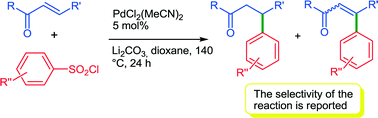
The reaction of benzenesulfonyl chlorides with enones was investigated. β-Ionone and benzalacetone in the presence of a palladium catalyst were found to afford the conjugate addition products instead of the expected Heck type products. The reaction tolerates a wide variety of substituents on the benzenesulfonyl chloride. It should be noted that no cleavage of the C–Br and C–I bonds was observed in the course of the reactions with 4-bromo- or 4-iodo-benzenesulfonyl chlorides, allowing further transformations. For example, using 4-bromobenzenesulfonyl chloride as the central unit, consecutive conjugate addition following arylations allowed access to substituted bi(hetero)aryls.

 Please wait while we load your content...
Please wait while we load your content...