Sporopollenin as an efficient green support for covalent immobilization of a lipase†
Abstract
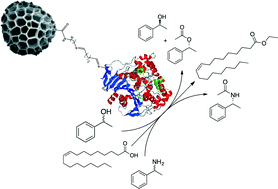
Sporopollenin exine capsules (SECs), derived from the spores of Lycopodium clavatum, have been functionalised with 1,n-diamines and the resulting aminoalkyl microcapsules used to immobilize Candida antarctica lipase B (Cal B) via a glutaradehyde-based diimine covalent linker. The supported enzyme efficiently catalyzes the esterification of oleic acid with ethanol. Initial rates using the SEC-CalBs were comparable to the commercial enzyme Novozym 435, but displayed up to 20-fold higher specific activity. The supported enzymes could also be recycled and after four cycles displayed only a modest decrease in conversions. In a kinetic resolution the SEC-CalBs efficiently acetylated rac-1-phenylethanol, with conversions up to 37% after 5 hours and product enantiomeric excesses of >99%. Related to this, the dynamic resolution of rac-1-phenylethylamine, in the presence of Pd–BaSO4 and ammonium formate, led to the acetylated amine with a 94% conversion and >99% ee.

 Please wait while we load your content...
Please wait while we load your content...