Electrocatalytic stereoselective transformation of aldehydes and two molecules of pyrazolin-5-one into (R*,R*)-bis(spiro-2,4-dihydro-3H-pyrazol-3-one)cyclopropanes†
Abstract
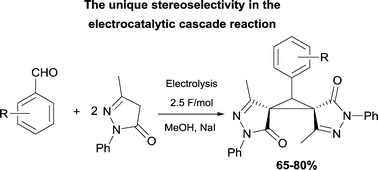
The electrocatalytic direct transformation of aldehydes and two molecules of pyrazolin-5-one in the presence of sodium iodide as a mediator in methanol in an undivided cell results in the stereoselective formation of substituted (R*,R*)-bis(spiro-2,4-dihydro-3H-pyrazol-3-one)cyclopropanes in 65–80% yields. Thus, a unique stereoselective cascade electrocatalytic process has been found. This novel electrocatalytic process opens an efficient and convenient way to synthesize (R*,R*)-bis(spiro-2,4-dihydro-3H-pyrazol-3-one)cyclopropanes – promising compounds for different biomedical applications. Thus, the simple mediatory system can initiate in an undivided cell under mild conditions the electrochemically induced cascade reaction of aldehydes with two molecules of pyrazolin-5-one.

 Please wait while we load your content...
Please wait while we load your content...