Improved three-way catalytic activity of bimetallic Ir–Rh catalysts supported on CeO2–ZrO2†
Abstract
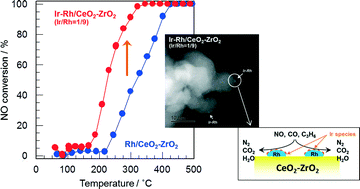
The addition of a small amount of Ir caused a significant increase in not only CO and C3H6 oxidation activity but also NO reduction activity of Rh/CeO2–ZrO2 with a Ce/Zr molar ratio of 1/4 for NO–CO–C3H6–H2–O2 reaction under stoichiometric conditions. The optimum Ir/Rh atomic ratio was 1/9. The Ir additive was found to suppress the preferential oxidation of CO and C3H6 by O2, leading to an improvement in the efficiency of CO and C3H6 utilization for NO reduction. Catalyst characterization such as H2-TPR and X-ray absorption spectroscopy (XANES and EXAFS) revealed that the Rh species in the Ir–Rh/CeO2–ZrO2 samples is mainly present as Rh2O3 nanoparticles and that the addition of a small amount of Ir (Ir/Rh = 1/9) can improve the reducibility of Rh2O3 nanoparticles. On the basis of surface analysis by XPS and FT-IR spectroscopy following CO adsorption and STEM observation, the formation of Ir–Rh nanoparticles composed of finely-divided Ir species on the surface of Rh particles with a size of 1 nm was considered to be responsible for the high catalytic activity of Ir–Rh/CeO2–ZrO2 for NO–CO–C3H6–H2–O2 reaction. Ir–Rh/CeO2–ZrO2 with Ir–Rh loading of 0.3 wt% still showed high catalytic performance, which is comparable with that of 0.5 wt% Rh/CeO2–ZrO2, minimizing Rh usage.

 Please wait while we load your content...
Please wait while we load your content...