Ag–Pd and CuO–Pd nanoparticles in a hydroxyl-group functionalized ionic liquid: synthesis, characterization and catalytic performance
Abstract
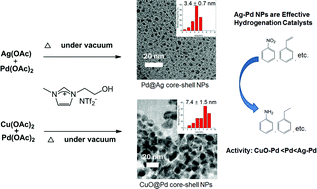
Heteronuclear Ag–Pd and CuO–Pd nanoparticles with a controllable Ag : Pd or Cu : Pd ratio were easily synthesized through thermal decomposition of their acetate salts in a functionalized ionic liquid, [C2OHmim][NTf2]. The structural and electronic properties of these particles were characterized by ICP-OES, TEM, XRD and XPS techniques. The Ag–Pd nanoparticles have a silver enriched core covered by a Pd enriched shell. The addition of Ag clearly enhanced the catalytic activity of Pd in the hydrogenation of –NO2 and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– functionalities. Interestingly, in the CuO–Pd nanoparticles, Pd tends to stay in the inner part of the particle, leaving the outer layer rich in CuO, which exhibits less activity than Pd NPs.
C– functionalities. Interestingly, in the CuO–Pd nanoparticles, Pd tends to stay in the inner part of the particle, leaving the outer layer rich in CuO, which exhibits less activity than Pd NPs.

 Please wait while we load your content...
Please wait while we load your content...