Solvent-free oxidation of dec-1-ene using gold/graphite catalyst using an in situ generated oxidant†
Abstract
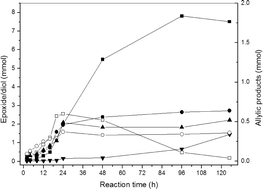
The oxidation of dec-1-ene is investigated under solvent-free conditions using gold nanoparticles supported on graphite and in a batch reactor in the presence of a radical initiator using oxygen from air as the terminal oxidant. The evolution of the products with reaction time shows that there is an initial induction period and during this time very little epoxide is fomed and the products of allylic oxidation are dominant. Subsequently the epoxide becomes the major product prior to the diol being formed from hydrolysis due to the presence of by-product water formed from the selective oxidation reaction. It is considered that the allylic oxidation products are in part converted in situ into aldehydes which form peracids during the induction period; the peracid leads to epoxide formation as the major product as the conversion is increased. The effect of addition of a number of aldehydes is investigated, all leading to enhanced epoxide formation when added in small amounts. Molar enhancements of epoxide yield can approach twice the amount of aldehyde initially added. This behaviour is in contrast to earlier studies which utilise aldehydes in greater than stoichiometric amounts as sacrificial reactants. The importance of in situ aldehyde formation is also demonstrated by the addition of benzyl alcohol which under the reaction conditions rapidly gives benzaldehyde and enhanced epoxide formation. Possible mechanistic interpretations of the observations are discussed.

 Please wait while we load your content...
Please wait while we load your content...