Catalytic activity of electrospun Ag and Ag/carbon composite fibres in partial methanol oxidation
Abstract
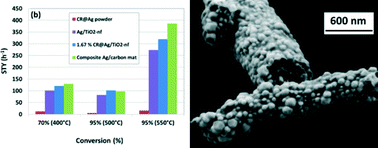
We report on the catalytic activity of Ag metal fibres and composite Ag/carbon fibres synthesized by electrospinning, in partial methanol oxidation (PMO) to formaldehyde. Uniform mats, containing fibres with 300–600 nm in diameter were prepared from a stable suspension of Ag nanoparticles (NP), AgNO3, PVA, water and ethanol. Thermal decomposition of the “green” mats was studied by TGA/DTA/MS in inert, strongly and mildly oxidizing atmospheres. Special heat profiles were developed to obtain metallic Ag as well as composite Ag/carbon (~10 wt.% carbon) fibre mats catalysts. The effect of sintering conditions on the morphology and catalytic activity were studied. Using a catalyst weight of 45–70 mg and a methanol WHSV in the range of 60–90 h−1, the highest conversion and selectivity for metallic Ag fibres at 550 °C were 94.2% and 82.4%, respectively. Under the same conditions, the catalytic activity for Ag/carbon mats was found to improve yielding a conversion and selectivity of 95.8% and 84.1%, respectively. Large space time yields (STY) of 56 h−1 and 64 h−1 were obtained for metal and metal/carbon cases, by optimizing the sintering conditions. Maximal WHSV and STY of 540 h−1 and 386 h−1 respectively were achieved with Ag/carbon composite catalyst at 95% conversion and 550 °C. In summary, we demonstrated the feasibility of electrospinning technology in producing highly active catalysts containing Ag and composite Ag/carbon fibres for partial oxidation of organic molecules. We also demonstrated the superior behaviour of the metal/carbon composite fibres over the metallic silver fibre and powder analogues.

 Please wait while we load your content...
Please wait while we load your content...