Preparation of graphene-supported highly dispersed nickel nanoparticles for the improved generation of hydrogen from ball-milled LiBH4
Abstract
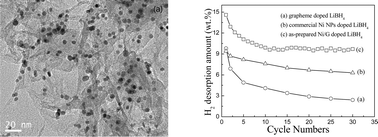
Using a hydrogen thermal reduction method, highly dispersed nickel nanoparticles (ca. 9.7 nm) were uniformly supported on graphene (Ni/G), and then ball-milled with LiBH4 to investigate in depth their hydrogen storage behaviour. The Ni/G-doped system exhibited high gravimetric hydrogen capacity, excellent rehydrogenation reversibility and rapid kinetics, due to the synergistic effect of nanoconfinement in graphene and catalysis by Ni nanoparticles, demonstrating a promising strategy for the effective design of hydrogen storage materials using LiBH4. In the case of doping with 20 wt.% Ni/G, thermal dehydrogenation of LiBH4 began around 180 °C, and the main hydrogen release peaks occurred at 275 and 465 °C, with a total weight loss of 15.2 wt.%. At 450 °C, about 12.8 wt.% hydrogen was desorbed in 45 min. More importantly, the hydrogen released during a second dehydrogenation remained at 12.6 wt.%, and a steady hydrogen capacity of ca. 9.8 wt.% was achieved during the thirtieth hydrogen uptake–release cycle under 3 MPa H2 at 400 °C, demonstrating the effectiveness of the Ni/G catalyst in the reversibility of hydrogen uptake of LiBH4 at relatively lower temperatures and pressure. During this process LiBH4 was re-formed and a new product, Li2B12H12, was detected following rehydrogenation.

 Please wait while we load your content...
Please wait while we load your content...