Heterogeneous catalysis for the direct synthesis of chemicals by borrowing hydrogen methodology
Abstract
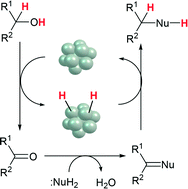
The development of heterogeneous catalysts for green chemical synthesis is a growing area in catalysis and green-sustainable chemistry. This review summarizes recent examples of hydrogen transfer-type reactions using supported transition metal catalysts with special emphasis on the direct synthesis of chemicals by borrowing hydrogen methodology. The highlighted reactions are: (1) transfer hydrogenation of carbonyl compounds, (2) transfer dehydrogenation of alcohols, (3) N-alkylation of amines or NH3 with alcohols, (4) alkylation of acidic CH2 or CH3 group with alcohols, (5) self- and cross-alkylation of alcohols, (6) N- or C3-alkylation of indoles with alcohols, (7) synthesis of quinolines from nitroarenes and alcohols, (8) synthesis of thioethers from thiols and alcohols, and (9) cross-alkylation of amines with different amines. These catalytic reactions have a common mechanistic feature: a domino dehydrogenation–condensation–hydrogenation sequence of alcohols (or amines) and nucleophiles. A key concept in the catalyst design in some of the systems is the multifunctionality of metal-loaded acidic and/or basic metal oxides, in which the acid and/or base sites selectively catalyze the condensation reactions, and the metal site catalyzes the transfer dehydrogenation of alcohol (or amines) and transfer hydrogenation of the condensation products as intermediates. In comparison with the recent examples of organometallic catalysis with borrowing hydrogen methodology, the advantages of heterogeneous catalysis are discussed in terms of catalytic efficiency and sustainability.

 Please wait while we load your content...
Please wait while we load your content...