Alternating ring-opening copolymerization of styrene oxide and maleic anhydride using asymmetrical bis-Schiff-base metal(iii) catalysts†
Abstract
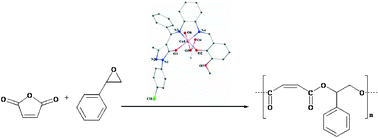
A ring-opening copolymerization of styrene oxide with maleic anhydride was performed by applying a series of asymmetrical bis-Schiff-base metal(III) catalysts. The chromium catalyst exhibited the best performance and therefore the catalyst (salen)CrCl (salen = 4-((Z)-(2-((E)-3,5-dibromo-2-hydroxybenzylideneamino)phenylimino)(phenyl)methyl)-1-(4-chlorophenyl)-3-methyl-1H-pyrazol-5(4H)-one) (7) was chosen for further studies. The investigations on the effect of different co-catalysts on the copolymerization of styrene oxide and maleic anhydride revealed that DMAP showed the highest activity, followed by PPN+Cl−, whereas Ph3P showed the lowest activity. 1H NMR and MALDI-TOF-MS spectra of the copolymers formed confirmed the alternating microstructures. The copolymerization of styrene oxide with maleic anhydride bearing a double bond in its structure was shown to be highly dependent on the polymerization condition, type of co-catalyst, monomer to catalyst ratio, temperature and time used in the copolymerization reaction. Applying chain transfer agents, alcohols, resulted in a decrease in molecular weight.

 Please wait while we load your content...
Please wait while we load your content...