How far can a single hydrogen bond tune the spectral properties of the GFP chromophore?†
Abstract
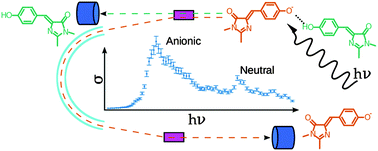
Photoabsorption of the hydrogen-bonded complex of a neutral and an anionic Green Fluorescent Protein chromophore has been studied using a new dual-detection approach to action-absorption spectroscopy. Following absorption of one photon, dissociation through a single channel ensures that the full absorption spectrum is measured. Our theoretical account of the spectral shape reveals that the anionic 0–0 transition (464 nm) is blue-shifted compared to that of the wild-type protein (478 nm) due to the stronger H-bond in the dimer, and represents an upper bound for that of the isolated anion. At the same time, the apparent effect of the H-bond for the neutral chromophore is as large as 0.5 eV, red-shifting the absorption maximum of the isolated neutral (340 nm) to that measured in the dimer (393 nm) and various proteins (∼395 nm). This shift results from changes in the topography of potential-energy surfaces in the Franck–Condon region of the H-bonded systems.


 Please wait while we load your content...
Please wait while we load your content...