Atomistic bond relaxation, energy entrapment, and electron polarization of the RbN and CsN clusters (N ≤ 58)
Abstract
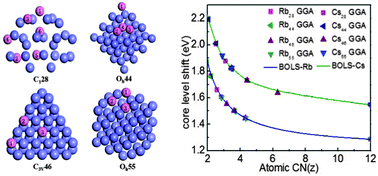
We systematically examined the effect of atomic undercoordination on the performance of bonds and electrons of Rb and Cs atomic clusters and their solid skins using a combination of photoelectron spectrometric analysis and density functional theory calculations. Results show that atomic coordination number reduction shortens the bonds by up to 30% for the Rb13 and Cs13 clusters, which densifies the local electrons and entraps their binding energies. Consistency between predictions and observations revealed that the Rb 4p level shifts from 13.654 eV for an isolated atom to a bulk value of 14.940 eV and the Cs 5p level shifts from 10.284 to 11.830 eV upon bulk formation. Such core–electron densification and entrapment polarize the valence charge from the inner to the outermost layer of skins, which perturbs the local Hamiltonian and hence dictates the unusual behavior of the Rb and Cs solid skins and nanocrystals.

 Please wait while we load your content...
Please wait while we load your content...