Chiral modification of platinum: ab initio study of the effect of hydrogen coadsorption on stability and geometry of adsorbed cinchona alkaloids†
Abstract
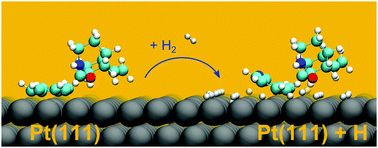
The cinchona alkaloids cinchonidine and cinchonine belong to the most efficient chiral modifiers for the noble metal-catalyzed enantioselective hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. Under reaction conditions these modifiers are coadsorbed on the noble metal surface with hydrogen. Using density functional theory, we studied the effect of coadsorbed hydrogen on the adsorption mode of cinchonidine and cinchonine on a Pt(111) surface at different hydrogen coverages. The theoretical study indicates that the presence of coadsorbed hydrogen affects both the adsorption geometry as well as the stability of the adsorbed cinchona alkaloids. At all hydrogen coverages the cinchona alkaloids are found to be adsorbed via anchoring of the quinoline moiety. In the absence of hydrogen as well as at low hydrogen coverage the quinoline moiety adsorbs nearly parallel to the surface, whereas at higher hydrogen coverage it becomes tilted. Higher hydrogen coverage as well as partial hydrogenation of the quinoline part of the cinchona alkaloid and hydrogen transfer to the C
C bonds. Under reaction conditions these modifiers are coadsorbed on the noble metal surface with hydrogen. Using density functional theory, we studied the effect of coadsorbed hydrogen on the adsorption mode of cinchonidine and cinchonine on a Pt(111) surface at different hydrogen coverages. The theoretical study indicates that the presence of coadsorbed hydrogen affects both the adsorption geometry as well as the stability of the adsorbed cinchona alkaloids. At all hydrogen coverages the cinchona alkaloids are found to be adsorbed via anchoring of the quinoline moiety. In the absence of hydrogen as well as at low hydrogen coverage the quinoline moiety adsorbs nearly parallel to the surface, whereas at higher hydrogen coverage it becomes tilted. Higher hydrogen coverage as well as partial hydrogenation of the quinoline part of the cinchona alkaloid and hydrogen transfer to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond at 10, 11 position of the quinuclidine moiety destabilize the adsorbed cinchona alkaloid, whereas hydrogen transfer to the nitrogen atom of the quinoline and the quinuclidine moiety stabilizes the adsorbed molecule. The stability as well as the adsorption geometry of the cinchona alkaloids are affected by the coadsorbed hydrogen and are proposed to influence the efficiency of the enantiodifferentiating ability of the chirally modified platinum surface.
C double bond at 10, 11 position of the quinuclidine moiety destabilize the adsorbed cinchona alkaloid, whereas hydrogen transfer to the nitrogen atom of the quinoline and the quinuclidine moiety stabilizes the adsorbed molecule. The stability as well as the adsorption geometry of the cinchona alkaloids are affected by the coadsorbed hydrogen and are proposed to influence the efficiency of the enantiodifferentiating ability of the chirally modified platinum surface.

 Please wait while we load your content...
Please wait while we load your content...