Determination of the thermodynamic activities of LiF and ThF4 in the LixTh1−xF4−3x liquid solution by Knudsen effusion mass spectrometry
Abstract
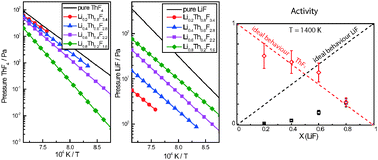
Knudsen effusion mass spectrometry (KEMS) has been used to investigate the vapour pressure over the molten LiF–ThF4 salt and determine the thermodynamic activity of LiF and ThF4 in the liquid solution. As part of the study, the vaporization of pure LiF and pure ThF4 was examined and the results were compared with the literature values finding a good agreement. Next, the vapour pressure of the LixTh1−xF4−3x liquid solution was investigated by measuring four samples having different compositions (XLiF ∼ 0.2, 0.4, 0.6, 0.8 mol%). In order to determine the thermodynamic activities, the vapour pressure of LiF and ThF4 species over the liquid solution, as calculated from our results, were compared with the vapour pressure over the pure LiF(l) and pure ThF4(l) systems. A strong deviation from the Raoult's law was observed, more evident in case of LiF species, in agreement with the predictions by our thermodynamic model.

 Please wait while we load your content...
Please wait while we load your content...