Quantum chemical analysis of thermodynamics of 2D cluster formation of alkanes at the water/vapor interface in the presence of aliphatic alcohols
Abstract
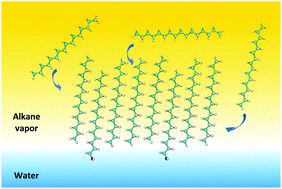
Using the quantum chemical semi-empirical PM3 method it is shown that aliphatic alcohols favor the spontaneous clusterization of vaporous alkanes at the water surface due to the change of adsorption from the barrier to non-barrier mechanism. A theoretical model of the non-barrier mechanism for monolayer formation is developed. In the framework of this model alcohols (or any other surfactants) act as ‘floats’, which interact with alkane molecules of the vapor phase using their hydrophobic part, whereas the hydrophilic part is immersed into the water phase. This results in a significant increase of contact effectiveness of alkanes with the interface during the adsorption and film formation. The obtained results are in good agreement with the existing experimental data. To test the model the thermodynamic and structural parameters of formation and clusterization are calculated for vaporous alkanes CnH2n+2 (nCH3 = 6–16) at the water surface in the presence of aliphatic alcohols CnH2n+1OH (nOH = 8–16) at 298 K. It is shown that the values of clusterization enthalpy, entropy and Gibbs' energy per one monomer of the cluster depend on the chain lengths of corresponding alcohols and alkanes, the alcohol molar fraction in the monolayers formed, and the shift of the alkane molecules with respect to the alcohol molecules Δn. Two possible competitive structures of mixed 2D film alkane–alcohol are considered: 2D films 1 with single alcohol molecules enclosed by alkane molecules (the alcohols do not form domains) and 2D films 2 that contain alcohol domains enclosed by alkane molecules. The formation of the alkane films of the first type is nearly independent of the surfactant type present at the interface, but depends on their molar fraction in the monolayer formed and the chain length of the compounds participating in the clusterization, whereas for the formation of the films of the second type the interaction between the hydrophilic parts of the surfactant is essential and different for various types of amphiphilic compounds. The energetic preference of the film formation of both types depends significantly on the chain length of compounds. The surfactant concentration (in the range of X = 0–10%) exerts a slight influence on the process of film formation.

 Please wait while we load your content...
Please wait while we load your content...