Molecular oxygen reduction catalyzed by a highly oxidative resistant complex of cobalt–hydrazone at the liquid/liquid interface†
Abstract
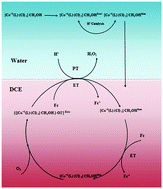
A new complex of Co(III) using an oxidative stable hydrazone ligand, CoL, was synthesized and characterized by elemental analysis, spectroscopic methods and single crystal X-ray analysis where HL is bis-[(E)-N′-(phenyl(pyridin-2-yl)methylene)]carbohydrazide. X-ray analysis revealed that the complex is mononuclear and the coordination environment around the Co(III) core is trans-[CoN4Cl2]. The catalytic activity of the complex in the oxygen reduction reaction was investigated. The complex is a highly oxidative resistant cobalt-hydrazone which can efficiently catalyze the reduction of oxygen (O2) by a weak electron donor ferrocene, (Fc), at the polarized water/1,2-dichloroethane (DCE) interface. Oxygen reduction is coupled with proton transfer from water to the organic phase to form hydrogen peroxide, which is extracted into the aqueous phase.


 Please wait while we load your content...
Please wait while we load your content...