Hydrogen bonding plays a significant role in the binding of coomassie brilliant blue-R to hemoglobin: FT-IR, fluorescence and molecular dynamics studies†
Abstract
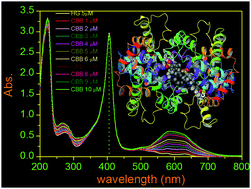
An analog of coomassie brilliant blue-R (CBB-R) was recently found to act as an antagonist to ATP-sensitive purinergic receptors (P2X7R) and has potential to be used in medicine. With the aim of understanding its transportation and distribution through blood, in this investigation, we measured the binding parameters of CBB-R with bovine hemoglobin (BHG). The molecule specifically bound to a single binding site of the protein with a stoichiometric ratio of 1 : 1 and the observed binding constant Ka was 3.5, 2.5, 2.0 and 1.5 × 105 M−1 at 20 °C, 27 °C, 37 °C and 45 °C, respectively. The measured respective ΔG0 values of the binding at four temperatures were −30.45, −22.44, −18.04 and −11.95 kJ mol−1. The ΔH0 (change in enthalpy) and ΔS0 (change in entropy) values were −23.6 kJ mol−1 and −70.66 J mol−1 respectively in the binding process. The negative value of ΔH0 and ΔS0 indicated that the binding of the molecule was thermodynamically favorable. The best energy structure in the molecular docking analysis revealed that CBB-R preferred to be intercalated in the cavity among the α2, β1 and β2 subunits and the binding location was 7.4 Å away from Trp37 in the β2 subunit. The binding of the molecule with the protein was stabilized by hydrogen bonds involving the side chain of two amino acid residues. The residues were Lys104 and Glu101 in the β2 subunit. The binding was further stabilized via hydrogen bond formation between the amide group of the peptide backbone (residue Tyr145 of the β1 subunit) and CBB-R. A shift of the amide I (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) band frequency of ∼8 cm−1 to low energy was ascribed to the hydrogen bond interaction involving the polypeptide carbonyl of the protein and the CBB-R molecule. In addition, two π–cation interactions between Lys99 of the α2 subunit and Lys104 of the β2 subunit and CBB-R contributed favorably in the binding processes. No substantial change in the soret and Q absorption bands of BHG could be observed in the presence of CBB-R. It indicated that the oxygen binding domain or the heme proximity was not blocked or substantially perturbed due to the binding of CBB-R. The circular dichroism and the molecular dynamics analysis further established that the binding interaction caused no significant alteration in the protein long range secondary structure.
O stretching) band frequency of ∼8 cm−1 to low energy was ascribed to the hydrogen bond interaction involving the polypeptide carbonyl of the protein and the CBB-R molecule. In addition, two π–cation interactions between Lys99 of the α2 subunit and Lys104 of the β2 subunit and CBB-R contributed favorably in the binding processes. No substantial change in the soret and Q absorption bands of BHG could be observed in the presence of CBB-R. It indicated that the oxygen binding domain or the heme proximity was not blocked or substantially perturbed due to the binding of CBB-R. The circular dichroism and the molecular dynamics analysis further established that the binding interaction caused no significant alteration in the protein long range secondary structure.

 Please wait while we load your content...
Please wait while we load your content...