Mechanical properties of monolayer sulphides: a comparative study between MoS2, HfS2 and TiS3
Abstract
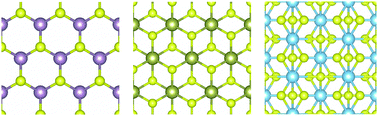
The in-plane stiffness (C), Poisson's ratio (ν), Young's modulus and ultimate strength (σ) along two different crystallographic orientations are calculated for the single layer crystals: MoS2, HfS2 and TiS3 in 1H, 1T and monoclinic phases. We find that MoS2 and HfS2 have isotropic in-plane stiffnesses of 124.24 N m−1 and 79.86 N m−1, respectively. While for TiS3 the in-plane stiffness is highly anisotropic due to its monoclinic structure, with Cx = 83.33 N m−1 and Cy = 133.56 N m−1 (x and y are parallel to its longer and shorter in-plane lattice vectors.). HfS2 which is in the 1T phase has the smallest anisotropy in its ultimate strength, whereas TiS3 in the monoclinic phase has the largest. Along the armchair direction MoS2 has the largest σ of 23.48 GPa, whereas along y TiS3 has the largest σ of 18.32 GPa. We have further analyzed the band gap response of these materials under uniaxial tensile strain, and find that they exhibit different behavior. Along both armchair and zigzag directions, the band gap of MoS2 (HfS2) decreases (increases) as strain increases, and the response is almost isotropic. For TiS3, the band gap decreases when strain is along x, while if strain is along y, the band gap increases first and then decreases beyond a threshold strain value. The different characteristics observed in these sulphides with different structures shed light on the relationship between the structure and properties, which is useful for applications in nanotechnology.

 Please wait while we load your content...
Please wait while we load your content...