Shikimic acid ozonolysis kinetics of the transition from liquid aqueous solution to highly viscous glass
Abstract
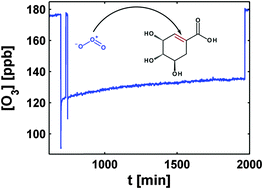
Ageing of particulate organic matter affects the composition and properties of atmospheric aerosol particles. Driven by temperature and humidity, the organic fraction can vary its physical state between liquid and amorphous solid, or rarely even crystalline. These transitions can influence the reaction kinetics due to limitations of mass transport in such (semi-) solid states, which in turn may influence the chemical ageing of particles containing such compounds. We have used coated wall flow tube experiments to investigate the reaction kinetics of the ozonolysis of shikimic acid, which serves as a proxy for oxygenated, water-soluble organic matter and can form a glass at room temperature. Particular attention was paid to how the presence of water influences the reaction, since it acts a plasticiser and thereby induces changes in the physical state. We analysed the results by means of a traditional resistor model, which assumes steady-state conditions. The ozonolysis rate of shikimic acid is strongly increased in the presence of water, a fact we attribute to the increased transport of O3 and shikimic acid through the condensed phase at lower viscosities. The analysis using the resistor model suggests that the system undergoes both surface and bulk reaction. The second-order rate coefficient of the bulk reaction is 3.7 (+1.5/−3.2) × 103 L mol−1 s−1. At low humidity and long timescales, the resistor model fails to describe the measurements appropriately. The persistent O3 uptake at very low humidity suggests contribution of a self-reaction of O3 on the surface.

 Please wait while we load your content...
Please wait while we load your content...