Even–odd product variation of the Cn+ + D2 (n = 4–9) reaction: complexity of the linear carbon cation electronic states†
Abstract
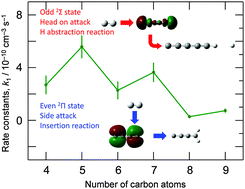
We have studied reactions between linear Cn+ (n = 4–9) and D2, using ion mobility mass spectrometry techniques and quantum chemical calculations in order to understand the complex reactivity of the linear cluster cations. Only linear CnD+ products were observed for the odd (n = 5, 7, 9) linear clusters, while CnD2+ was the main product for the even clusters. For the reaction rate constants determined for these two channels, we obtained the following two features: (1) the rate constant decreases with the size n, and (2) even-sized clusters have lower rate constants than neighboring odd-sized clusters. In the theoretical calculations using the CCSD(T) and B3LYP methods with the cc-pVTZ basis, we found that a low lying 2Σ state in odd clusters may play an important role in these reactions. This opposes the previous interpretation that the 2Πg/u state is the dominant electronic state for linear Cn+ (n = 4–9) clusters. We showed that a barrierless radical abstraction forming CnD+ occurs through a direct head on approach for the 2Σ state Cn+. In contrast, a carbene-like insertion forming CnD2+ occurs through a sideways approach for the 2Πg/u state Cn+. We have concluded that the higher rate constants for the odd clusters come from the existence of symmetry broken 2Σ states which are absent in even linear clusters.

 Please wait while we load your content...
Please wait while we load your content...