How do non-covalent complexes dissociate in droplets? A case study of the desolvation of dsDNA from a charged aqueous nanodrop†
Abstract
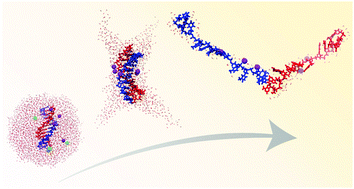
We present the desolvation mechanism of a double-stranded oligodeoxynucleotide (dsDNA) from an aqueous nanodrop studied by using atomistic molecular dynamics methods. The central theme of this study is the stability of a non-covalently bound complex, in general, and that of a dsDNA in particular, in a droplet environment. Among the factors that may affect the stability of a complex in an evaporating droplet we examine the increase in ion concentration and the distinct droplet morphologies arising from the charge-induced instability. We explore in detail a large set of aqueous nanodrops with excess negative charge, which comprise a dsDNA and Na+, Cl− ions at various concentrations. We find that for a square of the charge to volume ratio above that of the Rayleigh limit the droplet attains distinct “spiky” morphologies that disperse the charge in larger volume relative to that of the spherical drop. Moreover, it is found that it is possible for a non-covalent complex to remain associated in an unstable droplet as long as there is enough solvent to accommodate the instability. In the presence of Na+ and Cl− ions, the Na+ ions form adducts with the double helical DNA in the minor groove, which help stabilise the duplex state in the gas phase. The negative ions may be released from the droplet. In a DNA-containing droplet with a net charge that is less negative than 50% of the dsDNA charge, the DNA maintains a double-stranded state in the gas phase. Several of our findings are in good agreement with experiments, while the spiky droplet morphology due to the charge-induced instability calls for new experiments. The results shed light on the association properties of complexes of macromolecules in droplet environments, which are critical intermediates in electrospray ionisation experiments.

 Please wait while we load your content...
Please wait while we load your content...