U@C28: the electronic structure induced by the 32-electron principle†
Abstract
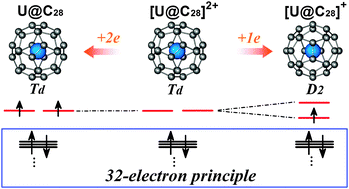
First principles calculations show that the neutral U@C28 has a (cage)2 ground state with Td symmetry instead of the long believed (5f)1(cage)1 ground state with D2 symmetry. Its 34 valence electrons preferentially obey the 32-electron principle which fills all the s-, p-, d-, and f-type valence shells of the uranium atom. The remaining two valence electrons cannot break the electronic configuration and thus are located on the cage.


 Please wait while we load your content...
Please wait while we load your content...