Structure study of a microemulsion system with an ionic liquid
Abstract
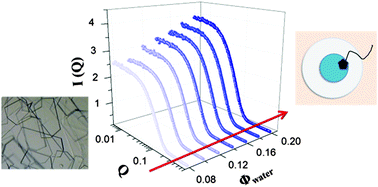
We found that an ionic liquid (IL) with a long alkyl chain moiety, 1-tetradecyl-3-methylimidazolium chloride (C14MIM·Cl), forms a single crystal after the addition of octanol in an alkane solvent. But the solution exhibits a structural change after adding a small amount of water. An optically clear solution is found within limits, and it is stable for several months. Since the IL molecule has an amphiphilic property, it behaves as a surfactant in the microemulsion system. But the IL formed a single crystal rather than a lyotropic liquid crystalline structure, unlike a typical surfactant. Therefore, it is important to understand the structure of the microemulsion system. We used the small angle neutron scattering (SANS) technique to investigate the structure. The scattering intensity was analyzed using a spherical core–shell model with the Schultz size distribution, and a contrast matching method was used to study the internal structure. The structure of the solution is confirmed to be a water-in-oil microemulsion system, and the swelling law is obeyed in the microemulsion system.

 Please wait while we load your content...
Please wait while we load your content...