Modulation of the aggregation properties of sodium deoxycholate in presence of hydrophilic imidazolium based ionic liquid: water dynamics study to probe the structural alteration of the aggregates†
Abstract
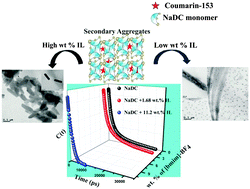
In this article, we have investigated the effect of a hydrophilic ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]-BF4), on the aggregation properties of a biological surfactant, sodium deoxycholate (NaDC), in water. In solution, unlike conventional surfactants it shows stepwise aggregation and the effect of the conventional ionic liquid on the aggregation properties is rather interesting. We have observed concentration dependent dual role of the ionic liquid; at their low concentration, the aggregated structure of NaDC reorganizes itself into an elongated rod like structure. However, the aggregated network is disintegrated into small aggregates upon further addition of ionic liquid. TEM (Transmission Electron Microscopy), SEM (Scanning Electron Microscopy) and FLIM (Fluorescence Lifetime Imaging Microscopy) images also confirmed the structural alteration of NaDC upon varying the concentration of the ionic liquid. The proton NMR data indicate that hydrophobic as well as electrostatic interaction is solely responsible for such structural adaptation of NaDC in the presence of an ionic liquid. The host–guest interaction inside the aggregates is monitored using Coumarin-153 (C-153) and the location of C-153 is probed by varying the excitation wavelength from 375 nm to 440 nm and the two binding sites of the aggregates are affected in a different fashion in the presence of ionic liquid. Excitation in the blue region selects the fluorophores which preferably bind to the buried region of the aggregates, whereas 440 nm excitation corresponds to the guest molecules which are exposed to the solvent molecules. The average solvation time of C-153 is increased in the presence of 1.68 wt% [bmim]-BF4 at λexc = 440 nm i.e. the probe molecules relocate themselves to a more restricted region. However, the average solvation time became 2.6 times faster in the presence of 11.2 wt% [bmim]-BF4, which corresponds to a more polar and exposed region. The time resolved anisotropy measurements and polarity determined by pyrene also supported our results in addition to solvation dynamics measurements. In summary, ionic liquids can modulate the host–guest interaction of bile salt aggregates, which can be used as nanocarriers for drug delivery.

 Please wait while we load your content...
Please wait while we load your content...