Effects of substituents on fluorometric detection of cyanide anions by indolium–coumarin dyads†
Abstract
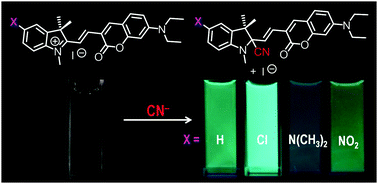
We synthesized an indolium–coumarin dyad (1) and its derivatives with –Cl (2), –N(CH3)2 (3), or –NO2 (4) substituent, and used them for fluorometric detection of cyanide anions (CN−) in aqueous media. All of the dyads exhibit fluorescence enhancement by CN−via a nucleophilic interaction of CN− with the indolium carbon atoms. Their fluorescence response and selectivity to CN−, however, depend strongly on the substituents. Ab initio calculation and kinetic analysis were performed to verify the behaviors. Substitution of electron-withdrawing groups (2 and 4) increases the electrophilicity of the indolium carbon. This decreases the activation enthalpy for the nucleophilic interaction with CN− and facilitates rapid CN− sensing. Compound 4 with very high electrophilicity, however, also promotes nucleophilic interaction with OH− in the solution, resulting in decreased CN− selectivity. As a result of this, the –Cl-substituted compound 2 containing the indolium carbon with appropriate electrophilicity facilitates rapid (within 1 min) and selective CN− detection.

 Please wait while we load your content...
Please wait while we load your content...