Peptides in the presence of aqueous ionic liquids: tunable co-solutes as denaturants or protectants?†
Abstract
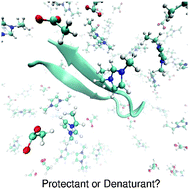
We studied the stability of a small β-hairpin peptide under the influence of an aqueous 1-ethyl-3-methylimidazolium acetate ([EMIM]+[ACE]−) solution via all-atom molecular dynamics simulations in combination with metadynamics. Our free energy results indicate a denaturation of the peptide structure in the presence of the ionic liquid which is validated by a significant broadening of the end-to-end distance. The radial distribution functions between the ions and the peptide were used for the calculation of the preferential binding coefficients in terms of the Kirkwood–Buff theory. A significant structure dependent binding behavior of acetate to the peptide was found which can be interpreted as the main reason for the denaturation of the native conformation. The outcomes of our simulations allow us to propose a simple mechanism to explain the unfolding of the peptide with regard to the specific properties of ionic liquids. Our results are in good agreement with experimental findings and demonstrate the benefits of ionic liquids as tunable co-solutes with regard to their influence on protein structural properties.

 Please wait while we load your content...
Please wait while we load your content...