Photoinduced water splitting via benzoquinone and semiquinone sensitisation
Abstract
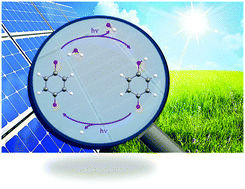
The splitting of water into H˙ and OH˙ radicals by sensitisation of a redox-active chromophore with sunlight may eventually become a viable way of producing unlimited, clean and sustainable energy. In this work, we explore the possibility of photo-oxidation of water via sensitisation of benzoquinone with ultraviolet (UV) light in the hydrogen-bonded complex of benzoquinone with a single water molecule. Using state-of-the-art quantum chemical calculations, the mechanisms of electron/proton transfer reactions between photoexcited benzoquinone and water are characterised. In the benzoquinone–H2O complex, photoexcitation of the chromophore leads to the population of locally excited ππ* and nπ* singlet states, which are coupled to hitherto unknown charge-transfer states. In the latter, an electron is transferred from the oxygen atom of the water molecule to the lowest π* orbital of benzoquinone. These charge-separated states drive the transfer of a proton from the water molecule to the carbonyl acceptor site, yielding the semiquinone–OH˙ biradical. Upon absorption of a second UV photon, the semiquinone radical may undergo O–H bond fission, which generates an H˙ radical and restores the benzoquinone photocatalyst. Our computational results shed light on long-standing questions regarding the nature of the photoreactive electronic states in the aqueous photochemistry of benzoquinone.

 Please wait while we load your content...
Please wait while we load your content...