Adsorption of 2-propanol on ice probed by ambient pressure X-ray photoelectron spectroscopy†
Abstract
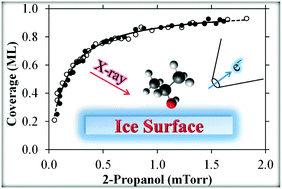
The interaction of 2-propanol with ice was examined via ambient pressure X-ray photoelectron spectroscopy (APXPS), a surface sensitive technique that probes the adsorbed 2-propanol directly with submonolayer resolution. Isothermal uptake experiments were performed on vapor deposited ice at 227 K in the presence of the equilibrium water vapor pressure of 0.05 Torr and 2-propanol partial pressures ranging from 5 × 10−5 to 2 × 10−3 Torr. The C 1s APXPS spectra of adsorbed 2-propanol showed two characteristic peaks associated with the COH alcohol group and CMe methyl groups in a 1 : 2 ratio, respectively. Coverage increased with 2-propanol partial pressure and followed first order Langmuir kinetics with a Langmuir constant of K = 6.3 × 103 Torr−1. The 1 : 2 ratio of COH : CMe remained constant with increasing coverage, indicating there is no chemical reaction upon adsorption. The observed Langmuir kinetics using APXPS is consistent with previous observations of other small chain alcohols via indirect adsorption methods using, e.g., Knudsen cell and coated wall flow tube reactors.

 Please wait while we load your content...
Please wait while we load your content...