Growth of Cu particles on a Cu2O truncated octahedron: tuning of the Cu content for efficient glucose sensing†
Abstract
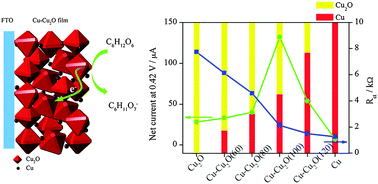
A simple and versatile hydrothermal method is developed to synthesize Cu–Cu2O, in which Cu particles grow on the surface of a Cu2O truncated octahedron. Through the reduction of Cu2+ by glucose in an alkaline solution, the Cu2O truncated octahedron is quickly formed via a kinetic control process, and then Cu particles selectively nucleate on the high-energy (110) facets of Cu2O, generating a heterostructure. The amount of Cu in the sample is successfully tuned by varying the reaction temperature. Compared to Cu2O, the hybrid Cu–Cu2O architecture shows superior electrocatalytic performance for glucose oxidation due to the synergistic effect between more electrocatalytic active but less conductive Cu2O and more conductive but less electrocatalytic active Cu. By tuning the content of Cu in the heterostructure, the highest electrocatalytic activity is achieved at the Cu/Cu2O molar ratio of 0.83.

 Please wait while we load your content...
Please wait while we load your content...