Distributed curvature and stability of fullerenes
Abstract
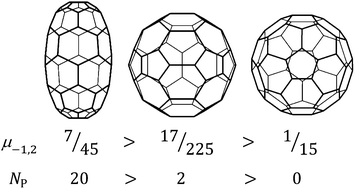
Energies of non-planar conjugated π systems are typically described qualitatively in terms of the balance of π stabilisation and the steric strain associated with geometric curvature. Curvature also has a purely graph-theoretical description: combinatorial curvature at a vertex of a polyhedral graph is defined as one minus half the vertex degree plus the sum of reciprocal sizes of the faces meeting at that vertex. Prisms and antiprisms have positive combinatorial vertex curvature at every vertex. Excluding these two infinite families, we call any other polyhedron with everywhere positive combinatorial curvature a PCC polyhedron. Cubic PCC polyhedra are initially common, but must eventually die out with increasing vertex count; the largest example constructed so far has 132 vertices. The fullerenes Cn have cubic polyhedral molecular graphs with n vertices, 12 pentagonal and (n/2 − 10) hexagonal faces. We show that there are exactly 39 PCC fullerenes, all in the range 20 ≤ n ≤ 60. In this range, there is only partial correlation between PCC status and stability as defined by minimum pentagon adjacency. The sum of vertex curvatures is 2 for any polyhedron; for fullerenes the sum of squared vertex curvatures is linearly related to the number of pentagon adjacencies and hence is a direct measure of relative stability of the lower (n ≤ 60) fullerenes. For n ≥ 62, non-PCC fullerenes with a minimum number of pentagon adjacencies minimise mean-square curvature. For n ≥ 70, minimum mean-square curvature implies isolation of pentagons, which is the strongest indicator of stability for a bare fullerene.

 Please wait while we load your content...
Please wait while we load your content...