Isotopic studies of the ammonia decomposition reaction mediated by sodium amide†
Abstract
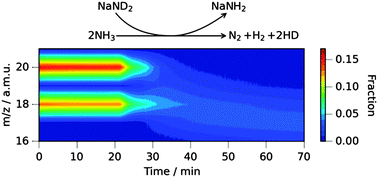
We demonstrate that the ammonia decomposition reaction catalysed by sodium amide proceeds under a different mechanism to ammonia decomposition over transition metal catalysts. Isotopic variants of ammonia and sodium amide reveal a significant kinetic isotope effect in contrast to the nickel-catalysed reaction where there is no such effect. The bulk composition of the catalyst is also shown to affect the kinetics of the ammonia decomposition reaction.

 Please wait while we load your content...
Please wait while we load your content...