Molecular dynamics modeling of carbon dioxide, water and natural organic matter in Na-hectorite
Abstract
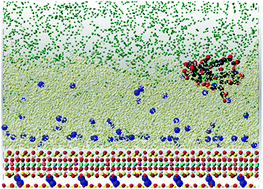
Molecular dynamics (MD) modeling of systems containing a Na-exchanged smectite clay (hectorite) and model natural organic matter (NOM) molecules along with pure H2O, pure CO2, or a mixture of H2O and CO2 provides significant new insight into the molecular scale interactions among silicate surfaces, dissolved cations and organic molecules, H2O and CO2 relevant to geological C-sequestration strategies. The simulations for systems containing H2O show the following results; (1) Na+ does not bridge between NOM molecules and the clay surface at protonation states comparable to near neutral pH conditions. (2) In systems without CO2 the NOM molecules retain charge balancing cations and drift away from the silicate surface. (3) In systems containing both H2O and CO2, the NOM molecules adopt equilibrium positions at the H2O–CO2 interface with the more hydrophilic structural elements facing the H2O and the more hydrophobic ones facing the CO2. In systems with only CO2, NOM and Na+ ions are pinned to the clay surface with the hydrophilic structural elements of the NOM pointed toward the clay surface. Dynamically, in systems with only CO2, Na+ diffusion is nearly eliminated, and in systems with a thin water film on the clay surface diffusion perpendicular the surface is greatly reduced relative to the system with bulk water. Energetically, the results for the systems with only H2O show that hydration of the net charge neutral Na–NOM molecule outweighs the sum of its Coulombic and dispersive interactions with the net charge-neutral Na-clay particle and the interactions of the water molecules with the hydrophobic structural elements of the NOM. The aggregation of NOM molecules in solution appears to be driven not by Na+ bridging between the molecules but by hydrophobic interactions between them. In contrast, for the systems with only CO2 the interaction between the Na–NOM molecules and the CO2 is outweighed by the interaction of NOM with the clay particle. With both H2O and CO2 present, the energetic interactions leading to the hydration of the Na-clay surface and the hydrophilic structural elements of the Na–NOM molecule and the hydrophobic interactions between the CO2 and the hydrophobic aromatic and aliphatic structural elements of the NOM can both be satisfied, leading to the Na–NOM molecules migrating away from the surface and residing at the H2O–CO2 interface. The MD results suggest some alternative explanations for the previously observed 23Na NMR behavior of Na-hectorite at elevated temperatures and CO2 pressures.

 Please wait while we load your content...
Please wait while we load your content...