Water-assisted self-photoredox of 2-(1-hydroxyethyl)-9,10-anthraquinone through a triplet excited state intra-molecular proton transfer pathway†
Abstract
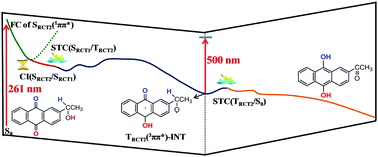
Using multi-configurational perturbation theory (CASPT2//CASSCF), a novel self-photoredox reaction for 2-(1-hydroxyethyl)-9,10-anthraquinone was proposed to effectively occur through two steps of triplet excited state intra-molecular proton transfer (ESIPT) reaction aided by water wires without the introduction of an external oxidant or reductant. The photoinduced charge transfer along the desired direction was determined to be the major driving force for the occurrence of the energetically favorable ESIPT in the triplet state, in which the water wires function as an effective proton relay and photocatalyst to lower the reaction barrier. The computational results provide convincing evidence that the deprotonation of the hydroxyl group in the triplet state and connecting water molecule(s) between that hydroxyl group and the carbonyl group that is protonated by a nearby water molecule in the water wire is the initial reaction step that triggers the protonation of the carbonyl group seen in the previously reported time-resolved spectroscopy experiments that produces a protonated carbonyl triplet intermediate that then undergoes a subsequent deprotonation of the methylene C–H in the triplet and ground states to complete the self-photoredox reaction of anthraquinone. Comparison of the theoretical results with previously reported results from time-resolved spectroscopy experiments indicate the photoredox reactions can occur either via a concerted or non-concerted deprotonation–protonation of distal sites of the molecule assisted by the connecting water molecules. These new insights will help provide benchmarks to elucidate the photochemistry of the anthraquinone and benzophenone compounds in acidic and/or neutral aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...