Spectroscopic evidence of ‘jumping and pecking’ of cholinium and H-bond enhanced cation–cation interaction in ionic liquids†
Abstract
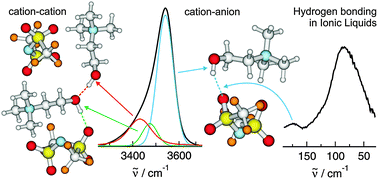
The subtle energy-balance between Coulomb-interaction, hydrogen bonding and dispersion forces governs the unique properties of ionic liquids. To measure weak interactions is still a challenge. This is in particular true in the condensed phase wherein a melange of different strong and directional types of interactions is present and cannot be detected separately. For the ionic liquids (2-hydroxyethyl)-trimethylammonium (cholinium) bis(trifluoro-methylsulfonyl)amide and N,N,N-trimethyl-N-propylammonium bis(trifluoromethylsulfonyl)amide which differ only in the 2-hydroxyethyl and the propyl groups of the cations, we could directly observe distinct vibrational signatures of hydrogen bonding between the cation and the anion indicated by ‘jumping and pecking’ motions of cholinium. The assignment could be confirmed by isotopic substitution H/D at the hydroxyl group of cholinium. For the first time we could also find direct spectroscopic evidence for H-bonding between like-charged ions. The repulsive Coulomb interaction between the cations is overcome by cooperative hydrogen bonding between the 2-hydroxyethyl functional groups of cholinium. This H-bond network is reflected in the properties of protic ionic liquids (PILs) such as viscosities and conductivities.

 Please wait while we load your content...
Please wait while we load your content...