Potentiometric and electrokinetic signatures of iron(ii) interactions with (α,γ)-Fe2O3
Abstract
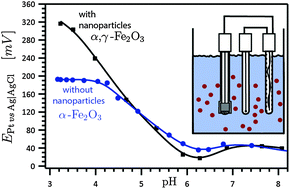
The electrochemical signatures of Fe(II) interactions with iron(III) oxides are poorly understood, despite their importance in controlling the amount of mobilized iron. Here, we report the potentiometric titration of α,γ-Fe2O3 oxides exposed to Fe(II) ions. We monitored in situ surface and ζ potentials, the ratio of mobilized ferric to ferrous, and the periodically analyzed nanoparticle crystal structure using X-ray diffraction. Electrokinetic potential reveals weak but still noticeable specific sorption of Fe(II) to the oxide surface under acidic conditions, and pronounced adsorption under alkaline conditions that results in a surface potential reversal. By monitoring the aqueous iron(II/III) fraction, we found that the addition of Fe(II) ions produces platinum electrode response consistent with the iron solubility–activity curve. Although, XRD analysis showed no evidence of γ-Fe2O3 transformations along the titration pathway despite iron cycling between aqueous and solid reservoirs, the magnetite formation cannot be ruled out.

 Please wait while we load your content...
Please wait while we load your content...