Formic acid oxidation on platinum: a simple mechanistic study†
Abstract
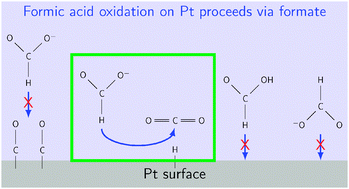
The oxidation of small organic acids on noble metal surfaces under electrocatalytic conditions is important for the operation of fuel cells and is of scientific interest, but the basic reaction mechanisms continue to be a matter of debate. Formic acid oxidation on platinum is one of the simplest of these reactions, yet even this model system remains poorly understood. Historically, proposed mechanisms for the oxidation of formic acid involve the acid molecule as a reactant, but recent studies suggest that the formate anion is the reactant. Ab initio studies of this reaction do not address formate as a possible reactant, likely because of the difficulty of calculating a charged species near a charged solvated surface under potential control. Using the recently-developed joint density functional theory (JDFT) framework for electrochemistry, we perform ab initio calculations on a Pt(111) surface to explore this reaction and help resolve the debate. We find that when a formate anion approaches the platinum surface at typical operating voltages, with H pointing towards the surface, it reacts to form CO2 and adsorbed H with no barrier on a clean Pt surface. This mechanism leads to a reaction rate proportional to formate concentration and number of available platinum sites. Additionally, high coverages of adsorbates lead to large reaction barriers, and consequently, we expect the availability of metal sites to limit the experimentally observed reaction rate.


 Please wait while we load your content...
Please wait while we load your content...