Low-energy electron interaction with retusin extracted from Maackia amurensis: towards a molecular mechanism of the biological activity of flavonoids†
Abstract
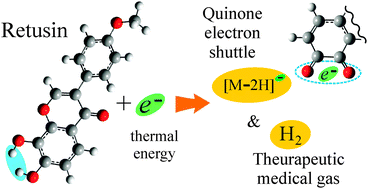
The antioxidant isoflavone retusin efficiently attaches low-energy electrons in vacuo, generating fragment species via dissociative electron attachment (DEA), as has been shown by DEA spectroscopy. According to in silico results obtained by means of density functional theory, retusin is able to attach solvated electrons and could be decomposed under reductive conditions in vivo, for instance, near the mitochondrial electron transport chain, analogous to gas-phase DEA. The most intense decay channels of retusin temporary negative ions were found to be associated with the elimination of H atoms and H2 molecules. Doubly dehydrogenated fragment anions were predicted to possess a quinone structure. It is thought that molecular hydrogen, known for its selective antioxidant properties, can be efficiently generated via electron attachment to retusin in mitochondria and may be responsible for its antioxidant activity. The second abundant species, i.e., quinone bearing an excess negative charge, can serve as an electron carrier and can return the captured electron back to the respiration cycle. The number of OH substituents and their relative positions are crucial for the present molecular mechanism, which can explain the radical scavenging activity of polyphenolic compounds.

 Please wait while we load your content...
Please wait while we load your content...