Disaggregation-induced fluorescence enhancement of NIAD-4 for the optical imaging of amyloid-β fibrils†
Abstract
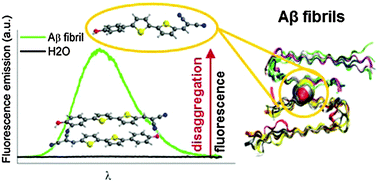
The main hallmark of Alzheimer's disease is the deposition of amyloid-β (Aβ) aggregates in the brain. An early diagnosis of the disease requires a fast and accurate detection of such aggregates in vivo. NIAD-4 is one of the most promising in vivo markers developed due to its high emission at λ > 600 nm and its ability to rapidly cross the blood–brain barrier (BBB) and target Aβ deposits. Furthermore, it shows a dramatic fluorescence enhancement upon binding to amyloid fibrils, which is essential for attaining good imaging contrast. Aiming at establishing novel design concepts for the preparation of optimized optical probes, the current work rationalizes the excellent performance of NIAD-4 by using a pool of computational (TD-DFT and CASPT2 calculations, ab initio molecular dynamics and protein energy landscape exploration) and spectroscopic techniques. Unlike other markers operating as molecular rotors or polarity-sensitive dyes, we uncover herein that the high fluorescence imaging contrast observed upon NIAD-4 binding to amyloid fibrils results from reversible aggregation. NIAD-4 forms non-emissive assemblies in aqueous solution already at very low concentrations, which convert into the highly fluorescent monomeric species by diffusion into the hydrophobic voids of Aβ deposits. This result paves the way to exploit aggregation-induced processes as a new strategy towards advanced fluorescence markers for amyloid detection.

 Please wait while we load your content...
Please wait while we load your content...